2025 Hybrid European Bioassay Conference
18th Annual | Rotterdam, Netherlands | 24-26 September 2025
Hilton Rotterdam Hotel
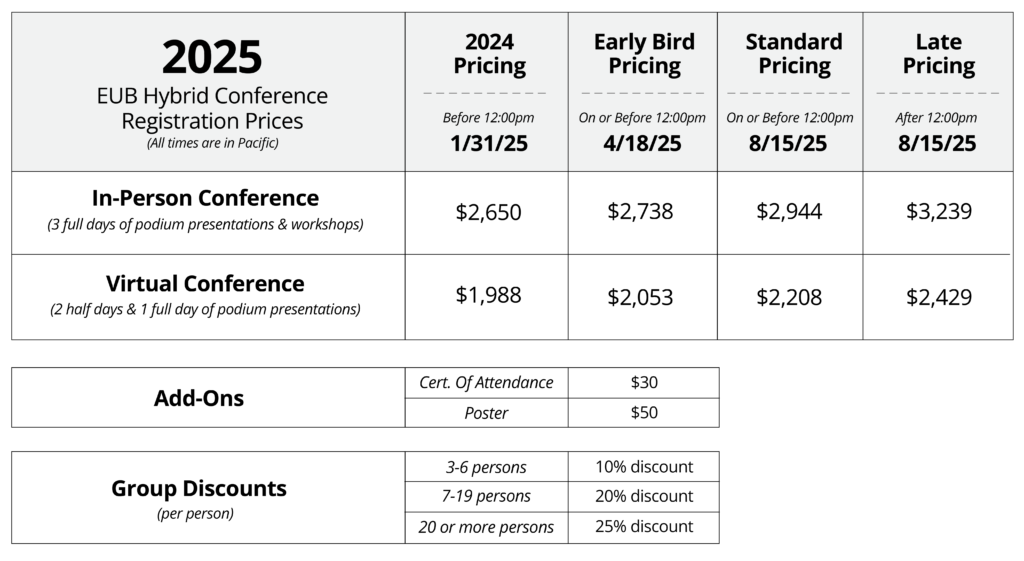
Registration
Registration is open for our Hybrid 2025 European Bioassay Conference.
In-person registration is SOLD OUT. Don’t miss out on our great talks! Register today and attend virtually!
Quick Links
24-26 September 2025
Rotterdam, Netherlands
Hybrid Conference
Agenda
Click below to view the draft agenda for our Hybrid 2025 European Bioassay Conference! Check the agenda for updates as we finalize our schedule.
Submit your speaker abstract or poster online through our website until 4 April 2025.
Participating Companies:
- Johnson & Johnson
- Sanofi
- Novartis
- Novo Nordisk A/S
- Roche
- Genmab
- GSK
- Baxalta Innovations
- Parexel
- Precision Bioassay Inc
- Voyager Therapeutics
- BioNTech SE
- Quantics
- Eurofins
- Promega
- acCELLerate
- Bioagilytix
- SVAR
- Charles River Labs
- Labcorp
- Stegmann Systems
- Bioassay GmbH
- QT Sense
