BEBPA Blog
Volume 2, Issue 6
Potency Method Commutability Study Leads To Late-Stage Bioassay Strategy Change: A Case of Unexpectedly High Sensitivity to Oxidation
By David Perez-Martinez, Senior Scientist, AstraZeneca
Strategy for Bioassay Development
Bioassay Development employs a phase-appropriate strategy to fulfil clinical and commercial regulatory requirements. This approach involves customizing the design and validation of assays according to the current stage of clinical development, adjusting the complexity and validation level to match the clinical trial phase. In the case of our drug modality, a fully reflective mode of action non-cell-based assay could in principle be submitted as late as the BLA stage, as the drug is intended to bind a soluble ligand, thus inhibiting the ligand-receptor interaction.
Given accelerated timelines between lead candidate optimization and the GMP campaign, a well established Competition Binding Assay format was proposed to support the manufacturing process control for First-Time in Human (FTIH) clinical lots, while concurrently developing a fully mode of action (MoA)-reflective non-cell-based Receptor Protein Competition Binding Assay for late-stage submission (see Fig. 1). However, this strategy faced challenges from a Health Authority (HA), which favoured a MoA-reflective Cell-Based Assay (CBA) for late-stage. As a result, the development of a new MoA reflective CBA was initiated to assess the drug’s performance within a biological system and its potential implications for our control strategy.
Late-Stage Filing Strategy: A MoA-reflective CBA for Characterization
Our hypothesis was centered on utilizing the new CBA for characterization and process comparability purposes while retaining the Receptor Protein Competition Binding Assay for late-stage routine quality control and avoiding a bridging study for the CBA to supplant the routine method. This would be achieved by showing that the two methods generate equivalent results across the whole working range of the assay. This was divided into equivalence testing using reference material and commutability testing across multiple lots, stress and as well as stability samples. To test our claim, we required evidence demonstrating performance equivalence between both MoA-reflective methods using reference material (mock linearity samples) prior to assessing commutability. Commutability aims to confirm that different sample types exhibit the same relative behaviour across two different potency methods as was observed with reference material. Both the routine and the new CBA methods (see Fig. 1) must fulfil full statistical equivalence criteria across a linear range and demonstrate complete commutability of samples from multiple lots, as well as stress and stability samples in order to demonstrate that the assays have equivalent performance.
We anticipated that our routine Receptor Protein Competition Assay would emerge as the most suitable release potency assay for late-stage and commercial phases. It fully reflects the MoA of binding of the drug to the ligand, resulting in the blockage of the ligand binding to the receptor on the cell surface. By showing comparable performance to the newly proposed CBA, this assay could potentially replace the need for a cell-based assay in routine quality control, offering advantages such as increased robustness, improved reproducibility, and reduced reagent costs.

Bioassay Strategy Change: CBA’s Unexpectedly High Sensitivity to Oxidation
The equivalence assessment between the new CBA and the Receptor protein competition assay demonstrated full equivalence across the 50% – 150% linear range between both methods (Pearson’s correlation r=0.97). Commutability could then be carried out because we had demonstrated equivalence across the whole working range with reference material. A combination of 18 lots selected based on the manufacturing process (P1, P2), material (DS, DP) and batch purpose (development, clinical and PPQ lots) were tested in both methods for use in commutability analysis. The samples tested from multiple lots met commutability criteria because all paired samples fell inside the 95%-prediction interval (PI) based on Deming regression. A stability study performed with 3 Process Performance Qualification lots (PPQ), 2 temperatures (25oC, 40oC) and 3 time points (1, 2 and 3 months) also met the commutability criteria. These study outcomes were expected for our drug modality. Analysis of the different stress studies (chemical oxidation, photo stress, high pH and heat stress) showed that the sample results from oxidative stress caused by photo stress and chemical oxidation (AAPH) did not show complete commutability between both methods (see Fig. 2).

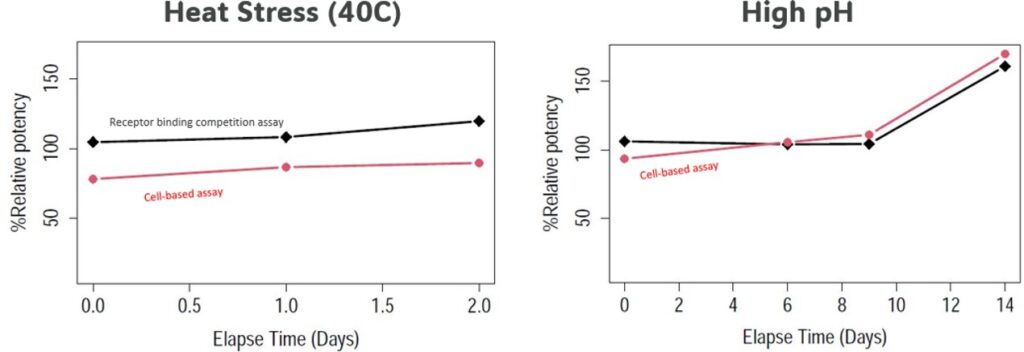
Trends of data from high pH and heat stress showed small differences in the dataset pattern over time (see Fig. 3) but they did meet the commutability criteria (Deming regression’s pair samples within 95% PI).
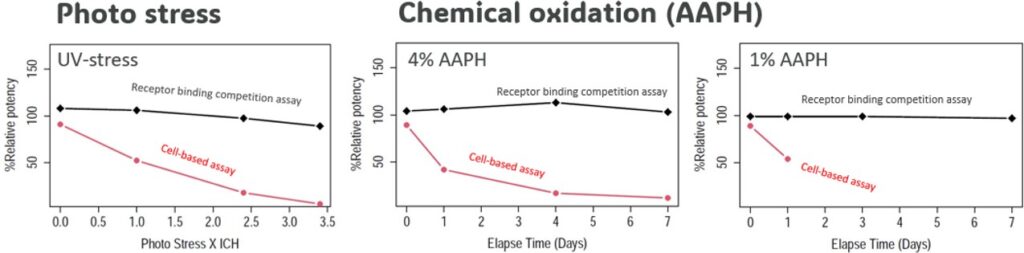
The oxidative stress studies revealed substantial response discrepancies between the two methods (see Fig. 4). The CBA demonstrated higher sensitivity to this type of stress than the Receptor Protein Competition Assay. The impact on the CBA’s % relative potency (%RP) was seen from the very first stress timepoint while the non-cell-based assay generated a flat trend under chemical oxidation. However, the Receptor Protein Competition Assay showed a shallow downward trend under photo stress but substantially different from the CBA one, which %RP value at 1X ICH was already close to falling outside of the qualified assay range (50% – 150%).
The results confirmed that the thorough design of the commutability study protocol yielded a scientifically rigorous outcome. The results of the equivalence and commutability study prompted a revision in the regulatory filing strategy. The CBA was now required to replace the routine Receptor Protein Competition Assay as a process control method. This change was due to the CBA’s greater sensitivity in detecting oxidative stress, which could be critical in measuring the effects of light exposure on the drug material, e.g. during the fill-finish manufacturing process.
A bridging analysis exercise was carried out using the data from the commutability study to assess the replacement of the Receptor protein competition assay with the CBA and demonstrate acceptable performance by meeting our preset method bridging criteria. The Bridging study results showed performance equivalence, aside from oxidative stress, between the new CBA and the routine Receptor Protein Competition Assay.
Conclusions and Lessons
In summary the commutability study showed that we could not defend the use of the competition binding assay for commercial in favour of the new cell-based assay developed as a characterization method for our control strategy to show the impact of several degradation pathways in a biological system.
Finally, lessons were learnt for future regulatory submissions. We recognized the importance of proactive internal planning to assess potential liabilities early, as well as the implementation of potency and characterization assays to identify these risks, as key activities in our product life-cycle planning.

About The Author: David Perez-Martinez
Dr. David Perez-Martinez has an extensive background developing non-cell-based assays and serving as a representative for Bioassay in CMC Project Teams within the Cambridge Bioassay Development Team. In addition, he has held managerial positions for both lab quality and personnel.
