BEBPA Blog
Tech Briefing: Relative Potency Assays: Parallel Line vs. Slope Ratio Assays
By Laureen Little, Principal Consultant, Quality Services and President (BEBPA)
Although the potency bioassay is considered a critical analytical method, finding regulatory guidelines which answer practical questions can be difficult. The pharmacopeia, USP and EP have the most prescriptive documents.
The USP has three chapters numbered:
<1032> Design and Development of Biological Assays
<1033> Biological Assay Validation
<1034> Analysis of Biological Assays
Steven Walfish, currently of Iovance and formerly with the USP has written an excellent review of these documents and their role in bioassay development and validation. (1)
Additionally, the EP has a chapter on the statistical analysis of bioassays.
Chapter 5.3 Statistical Analysis of Results of Biological Assays and Test.
Both of these chapters are extremely useful, especially for those scientists with a limited experience of relative potency assays. Most analytical scientists are trained in either reading an unknown sample result off a standard curve, or comparing areas under the curve. Relative potency bioassays, however, compare the dose-response curve of a test sample versus the dose-response curve of a standard preparation. If the assay is a parallel line assay (look at the X axis – is it a log function?) one looks for a right or left shift of the curve.
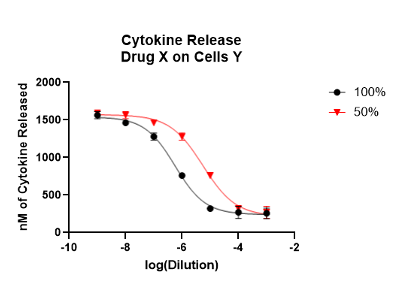
If the assay is a slope ratio assay (look at the X-axis if it is a function of the drug concentration without logging) then one compares the slopes of the dose-response curve of the test sample vs. the dose-response curve of the reference samples.

Needless to say, this is a drastic over simplification of the rigorous and appropriate analysis of potency assay results. I strongly urge you to read the four pharmacopeial chapters listed above.
While these chapters are a great help, they often do not address some of the nitty gritty issues surrounding the development of a potency assay. These questions include: Does my assay sufficiently reflect the mechanism of action of my therapeutic? What do the regulators expect? What do I do if we (the developers) disagree with regulatory counterparts? What is a phase appropriate assay for releasing clinical material? How does this differ from the requirements for a commercial product?
There are some product specific and other potency guidances, include:
- Potency Assurance for Cellular and Gene Therapy Products draft guidance issued December 2023, available here.
- Potency Assay Considerations for Monoclonal Antibodies and Other Therapeutic Proteins Targeting Viral Pathogens issues March 2023, available here.
- ICH Q2(R2) Validation of analytical procedures – Scientific guideline, effective June 14, 2024 now includes an example for biologic assays, specifically ELIS, SPR or cell-based assay for determining potency.
Still if you are responsible for developing the bioassays, you will not find all of the critical information about regulatory expectations in these guidances. My best recommendation is that you attend the upcoming BEBPA Hybrid European Bioassay Conference. This is our 18th annual conference and the information being shared is getting better every year. Some of the highlighted talks this year include:

A Case Study of a Potency Assurance Strategy: How Scientific Data Convinced Health Authorities to Accept an Alternative Proposal
Femke de Keijzer-van de Water
Janssen Vaccines & Prevention BV (part of Johnson & Johnson)

Analytical and Regulatory Challenges in Developing Potency Assays for Cell Therapies Targeting Rare Diseases
Alicja Fiedorowicz
Dark Horse Consulting

Practical Aspects of Validating Precision and Accuracy According to the ICH Q2 (R2)
Lasse Wæhrens
Novo Nordisk A/S

Tools and Designs for Evaluation of Potency: Examples from the Practice
Sonja Klingelhöfer
Richter BioLogics GmbH
These are just a few of the talks. To see our ever-evolving agenda look here. We are adding new talks and more details about the accepted talks every day.
This 2025 event is happening in Rotterdam, Netherlands on 24 to 26 September 2025. This conference does sell out – so please sign up today!
References
- The Role of USP Chapters in Bioassay Development and Validation Steven L. Walfish, Principal, Iovance, BEBPA Blog, Volume 1, Issue 10, https://bebpa.org/the-role-of-usp-chapters-in-bioassay-development-and-validation/
- EP chapter 5.3. Statistical Analysis of Results of Biological Assays and Test, https://www.scribd.com/document/691082158/5-3-STATISTICAL-ANALYSIS-OF-RESULTS-OF-BIOLOGICAL-ASSAYS-AND-TESTS
