BEBPA Blog
Reference Material for Late Stage Potency Assay Development
Reference material is a critical reagent for nearly all CMC release assays for biotherapeutic products. Potency assays are particularly reliant upon the use of a reference standard in the daily use of the assay. As a relative assay, potency is both assigned from and assessed for similarity to the dose-response curve of the reference during each run. Thus, if you get the reference material even slightly wrong, you will experience more aberrant Out-of-Specification Results, more system suitability failures and an overall decrease in the manufacturability of the product.
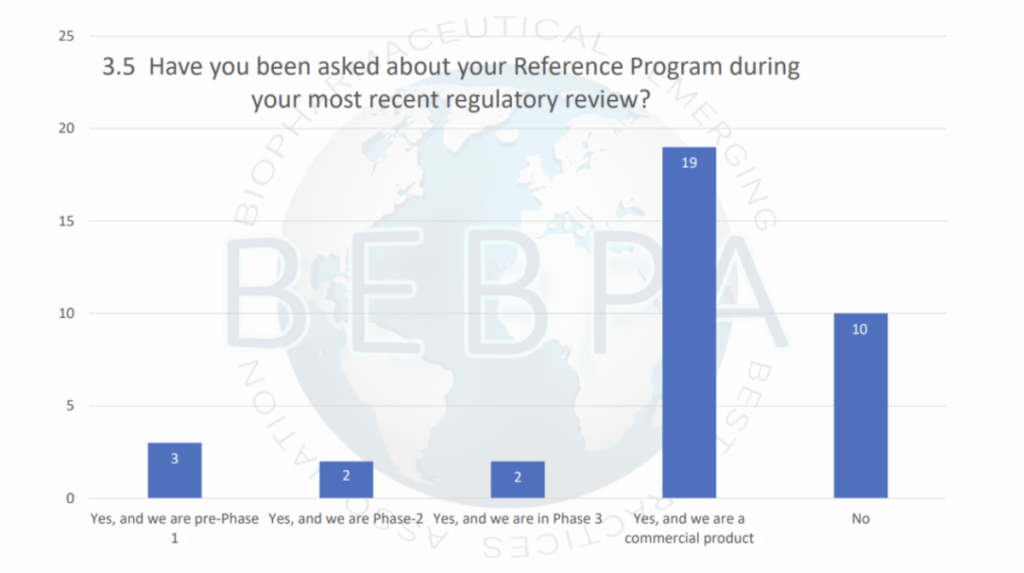
Because of its critical role, reference material questions are routinely asked about during regulatory reviews. In a recent audience poll at the BEBPA Bioassay Conference, more than 2/3 of the attendees reported that during their most recent regulatory review a question was asked about the reference material.
Many of these questions were asked during late stage of product development or after approval. This scrutiny is a reflection of the myriad of problems which can happen during late-stage product development.
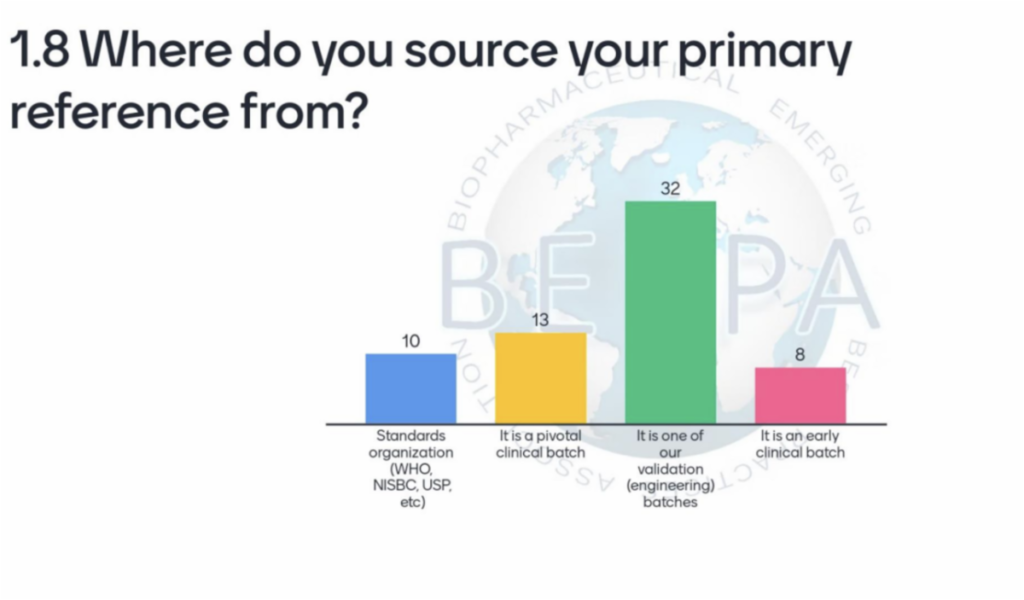
Despite the rise of biosimilar products, we find that most of us do not have available international references, and therefore, use either an engineering lot or a pivotal clinical batch for our first reference. See our poll results below.
However, probably the most interesting trend that we see from our more recent polls concerns the implementation of two-tiered reference programs. A two-tiered system consists of a primary (aka gold standard) reference and a secondary (aka working) product reference. The working reference typically comes from the standard manufacturing stream and is qualified against the master reference material. It is used on a daily basis to release product. The primary reference material is that material which comes from the pivotal clinical or engineering batch and is used to qualify secondary (working) references and/or for comparability studies when changing the manufacturing process. Several papers have been published in the past several years extolling the virtues of such a two-tiered reference programs. (See the reference list below).
In 2019, we asked bioassay conferences attendees if they use a two-tier reference system and found that 50% of the audience had only a working reference material – compare this to March 2023 in the US in which 28% stated they only had one-tier systems. Perhaps practices are changing? We will be polling the audience again this year – join us at our upcoming 2023 EUR Bioassay Conference in Bled, Slovenia to find out if this is a trend – or just an example of small sample size statistics
Also at this conference, we will have a half-day workshop dedicated to the topic of late-stage potency assays with a focus on reference material. This in-person attendees only workshop is entitled:
Care and Feeding of a Late-Stage Potency Assay
This two-part workshop covers two critical topics for the debut of late-stage potency assay into a commercial environment: 1) Method transfer and 2) The establishment of potency assay product reference material. Specifically, we will discuss:
Part 1: Assay Transfer
• Assay preparedness for method transfer
• Types of method transfer including covalidation, transfer protocol and other approaches
• Assay monitoring data and the interim system suitability criteria to ease transfer.
Part 2: Reference Material
• Selection of reference material for BLAs and initial years of product approval
• Qualification of reference material
• Requirements for monitoring the reference material
This workshop is presented by Dr. Sian Estdale, Labcorp, Dr. Alex Knorre, Eurofins BioPharma Product Testing, Dr. Laureen Little, Quality Services
This is just one of the workshops from our line-up of over 20 talks and 6 workshops and interest groups. (See the complete agenda HERE). Last year we sold out for the onsite conference, and are on track to have even more attendees this year. Please don’t miss out and register HERE. The podium presentations are available virtually and on-site, however, our workshops and discussion groups are only available to on-site attendees. Don’t miss this opportunity to hear great talks, network with your colleagues and keep up with the industry happenings and trends.
Reference Materials Reading list:
General Guidance
Recommendations and Best Practices for Reference Standards and Reagents Used in Bioanalytical Method Validation. Joseph F. Bower, Jennifer B. McClung, Carl Watson, Takahiko Osumi, and Kátia Pastre. The AAPS Journal 2014 Mar, 16(2): 352-356 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3933579/
Reference Standards for Therapeutic Proteins: Current Regulatory and Scientific Best Practices (Part 1). MireSluis A, Ritter N., Cherney, B., Schmalzing, D., and Blumel M. BioProcess Intl., March 1, 2014 https://bioprocessintl.com/upstream-processing/assays/reference-standards-for-therapeutic-proteins-350518/
Reference Standards for Therapeutic Proteins (Part 2). Mire-Sluis, A. BioProcess Intl. May 1, 2014 https://bioprocessintl.com/2014/reference-standards-for-therapeutic-proteins-351584/
World Health Organization, Expert Committee on Biological Standardization. Sixty sixth report. Report of a WHO informal consultation on international standards for biotherapeutic products. WHO Technical Report series 999:13–15, 2016. http://apps.who.int/iris/bitstream/10665/208900/1/9789240695634-eng.pdf
Stability of Reference Material:
Rapid optimization of protein freeze-drying formulations using ultra scale-down and factorial design of experiment in microplates. Grant Y, Matejtschuk P, Dalby PA. Biotechnol Bioeng. 2009 Dec 1;104(5):957-64. https://www.ncbi.nlm.nih.gov/pubmed/19530082
The International Reference Preparation of Tetracosactide for Bioassay: characterization and estimation of its (1-24)corticotrophin-tetracosapeptide content by physicochemical and biological methods. Storring PL, Witthaus G, Gaines Das RE, Stamm W. J Endocrinol. 1984 Jan;100(1):51-60. “Bioassay estimates of samples … which had undergone significant degradation were higher than estimates by HPLC.” https://www.ncbi.nlm.nih.gov/pubmed/6317783
