BEBPA Blog
Tech Briefing: Ins and Outs of Data Analysis
Establishing a potency assay to release a biopharmaceutical product is challenging in that we are attempting to harness biology (cells, enzymes, biomarkers, etc) to give us reliable and accurate values. Naively, as bioassay scientists, we often think this is what sets us apart from other, more “simple”, physical/chemical analyses. We work very hard and may accomplish getting a nice, satisfying dose-response curve:
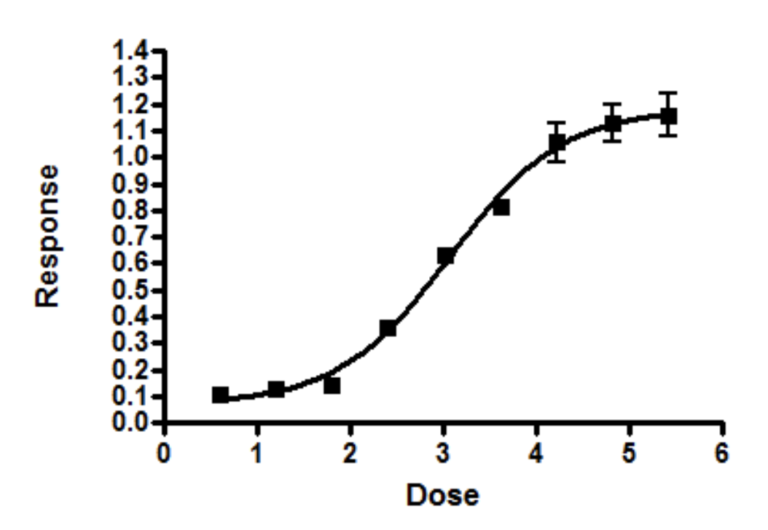
Just when we feel our work is done, we turn our attention to how to interpret the dose response curves and realize that the complications have just begun. How do we go from multiple dose-response curves, such as the reference dose-response curve vs. the test dose-response curve, to calculating a potency result with confidence intervals!
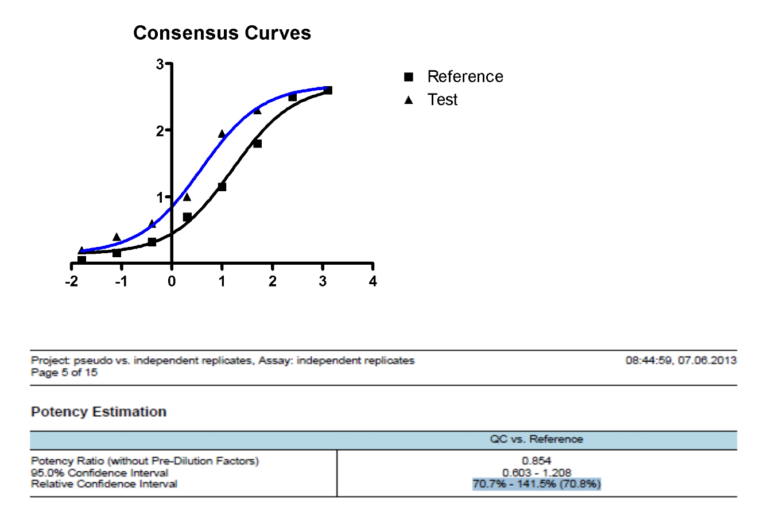
We often hear from our colleagues that we need to watch out for pseudo-replicates, ensure we do not over-rely on R² for assessing model fit, and that we can use outlier analyses, and we really should use equivalence testing rather than difference testing…..and so on and so on. This makes us yearn for the days of simply looking for a nice dose-response curve! This data analysis step presents many stumbling blocks for the bioassay developer. Such questions often include:
- What is dose-response similarity? How dissimilar may two curves be, before we can no longer calculate potency.
- What if we cannot saturate the assay and cannot define an upper asymptote on the dose-response curve?
- Do we run replicates within the assay, and if we do, should we be averaging them?
- How do we handle outliers within the assay?
During the March 2023 US Bioassay Conference in Long Beach, CA (See conference brochure here), we have planned an afternoon interest group (in-person attendees only) which touches specifically on these topics. Speakers include:
Partial Dose-Response Curves – Contributions To The Discussion On “Allowed” Non-Similarity In Biological Assays
Ralf Stegmann, CEO, Stegmann Systems
Replicates: Should They Be Averaged Before Modelling?
Matthew Stephenson, Director of Statistics, Quantics Biostatistics
Poster Presentation: Outlier Analysis for Relative Potency Assays Using SoftMax Pro Function for Rosner Extreme Studentized Deviate Test
Alena Nikolskaya, Associate Director, Abzena
Using Equivalence Testing to Define Lot Release Limits for Product Potency
Nancy Sajjadi, Founder and Principal Consultant, Sajjadi Consulting
All You Ever Wanted to Know About Target Measurement Uncertainty and Total Analytical Error for Analytical Validation
Pierre Lebrun, Director Statistics, Pharmalex Belgium SA
Limiting Potency Bias from Allowed Non-Similarity While Protecting the Similarity Pass Rate
David Lansky, President
These session will provide answers to many of our data handling questions and give us insight into how our peers are solving these problems.
The BEBPA Bioassay Conferences are known for its practical hands-on talks, sharing of critical hints and fabulous scientific discussions. At the 2024 US Bioassay Conference, we will be doing a deep dive into data analysis issues, as well as handling/developing potency assay reference material and potency assays for complex products. Don’t wait too long to sign up for this conference as it does sell out.
